GOUT: THE MOST COMMON INFLAMMATORY ARTHRITIS IN THE UNITED STATES
Affects an estimated 14 million adults in the United States.
An estimated approximately 5% of the gout population is considered to have chronic refractory disease. Patients with other severe forms of gout may also benefit from new therapies.
Cause
Uncontrolled gout occurs when urate-lowering therapies (ULTs) fail to lower serum uric acid (sUA) levels below the gout guideline goal of 6 mg/dL at the maximum medically appropriate dosage or when gout does not improve clinically with oral ULTs. Uncontrolled gout is also reported to involve urate crystal deposition and associated inflammation in joints, soft tissues and organs. Currently available ULTs can be effective in treating gout. However, low adherence, under dosing and disease progression that cause high patient burden require, we believe, new, effective and safe therapies to treat these underserved uncontrolled gout patients.
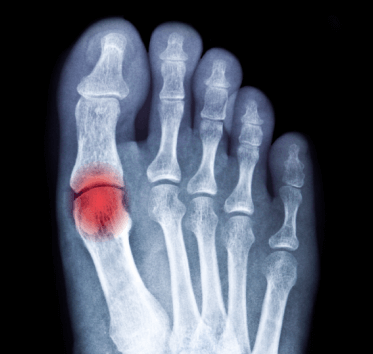
Uncontrolled Gout
The risk of gout increases with age, and it is thus more common in ageing populations. Gout results from sustained elevation of serum urate levels (hyperuricaemia). Urate levels may increase due to diet, genetic predisposition and environmental factors leading to the deposition of monosodium urate crystals, which triggers recurrent episodes of pronounced acute inflammation, known as gout flares. Gout leads to substantial morbidity, severe pain, reduced quality of life, decreased physical function, increased healthcare costs, and lost economic productivity. In addition, in some patients deposits of monosodium urate crystals under the skin form Tophi on joints, tendons and other soft tissue, which can cause inflammation, pain and deformation. Furthermore, gout is strongly associated with a number of comorbidities, including hypertension, cardiovascular disease, renal impairment, diabetes, obesity, hyperlipidaemia and frequently in a combination known as the metabolic syndrome.

PRX-115
A recombinant PEGylated uricase enzyme, chemically modified enzyme in development for the potential treatment of uncontrolled gout.PRX-115 is our recombinant PEGylated uricase (urate oxidase) – a chemically modified enzyme under development for the potential treatment of patients with uncontrolled gout. The uricase enzyme, that does not exist naturally in humans, converts uric acid to allantoin, which is easily eliminated through urine . This recombinant enzyme, expressed via our ProCellEx system, is designed to lower uric acid levels and improve clinical manifestation of the disease while having low immunogenicity and increased half-life of the drug in the blood.
Pre-clinical data demonstrates long half-life, reduced immunogenic risk and high specific activity which supports the potential of PRX-115 to be a safe and effective treatment for patients with uncontrolled gout. One-month multiple dosing toxicity studies in two species and 6-month multiple dosing toxicity study in one specie were conducted to support single and multiple dose studies in humans.
Clinical Development
PRX-115 was studied in a phase I First in Human (FIH) single ascending dose clinical trial to evaluate its safety, pharmacokinetics, pharmacodynamics and immunogenicity in patients with elevated uric acid levels. It was a double blind, placebo-controlled study in which 64 randomized subjects were enrolled across eight cohorts; 48 subjects were treated with PRX–115 and 16 subjects were treated with placebo.
Key results are as follows:

Exposure to PRX–115 increased in a dose–dependent manner, detectable PRX–115 levels were observed in plasma for up to 12 weeks from subjects in cohorts 6, 7 and 8 in the high dose cohorts

PRX–115 rapidly reduced plasma uric acid levels to below 6.0 mg/dL over time following a single administration at the highest doses

All randomized participants completed the study; PRX–115 was found to be well-tolerated
For more information, see our poster presentation from the ACR Convergence 2024.
The results demonstrate that PRX-115 may offer an effective uric acid-lowering treatment with an added benefit of a potentially wide dosing interval, which may enhance patient compliance and treatment flexibility. Further studies are needed to confirm the long-term safety and efficacy of PRX-115 in the gout patient population.
We have initiated preparations for a phase II clinical trial of PRX-115, and we expect to commence the study in the second half of 2025.

