Pegunigalsidase alfa (PRX-102): In development for the treatment of Fabry Disease
About Fabry Disease
Fabry disease is an X-linked inherited disease that results from abnormal deposits of a fatty substance called globotriaosylceramide (Gb3) in blood vessel walls throughout a person’s body. Fabry disease occurs in one person per 40,000. Fabry patients inherit a deficiency of the enzyme alpha-galactosidase-A, which is normally responsible for the breakdown of Gb3. The abnormal storage of Gb3 increases with time and, accordingly, Gb3 accumulates, primarily in the blood and in the blood vessel walls. The channels of blood vessels narrow, leading to decreased blood flow and decreased tissue nourishment. The ultimate consequences of Gb3 deposition range from episodes of pain and impaired peripheral sensation to end-organ failure—particularly of the kidneys, but also of the heart and the cerebrovascular system. Fabry disease is generally treated with enzyme replacement therapy (ERT), meaning the replacement of the missing alpha-Galactosidase-A enzyme with a recombinant form of the protein via intravenous infusion once every two weeks.
About pegunigalsidase alfa
Pegunigalsidase alfa is designed to be a plant cell culture-expressed, and a chemically modified version of, the recombinant alpha-Galactosidase-A protein. Protein sub-units are covalently bound via chemical cross-linking using PEG chains, resulting in a more active and stable molecule compared to the current available versions of the molecule as seen in preclinical models. In clinical research, pegunigalsidase alfa appears to have a favorable circulatory half-life, with targeted enzyme activity in organs affected by Fabry disease.
Based on our clinical data to date, we believe that these characteristics may potentially enable a treatment option of once-monthly infusions as opposed to bi-weekly infusions.
We are developing pegunigalsidase alfa in two dosing regimens with the goal of meeting two important, unmet needs:
- Demonstrating benefit for Fabry patients with declining renal function
- Lowering the treatment burden of bi-weekly infusions
In the BALANCE study we are currently evaluating the safety and efficacy of pegunigalsidase alfa 1mg/kg dosed every 2 weeks and assessing its effects in Fabry patients with declining renal function versus the currently used enzyme replacement therapy, Fabrazyme®.
For more information on the BALANCE study, please click here (Enrollment Completed).
In the BRIDGE study, we are currently evaluating the safety and efficacy of pegunigalsidase alfa 1mg/kg dosed every 2 weeks in Fabry patients previously treated with Replagal®
For more information on the BRIDGE study, please click here (Study Completed).
In the BRIGHT study, we are evaluating the safety and efficacy of pegunigalsidase alfa 2mg/kg, dosed once every 4 weeks, and assessing whether patients maintain clinical stability after being switched to this regimen from currently used enzyme replacement therapy regimens dosed bi-weekly.
For more information on the BRIGHT study, please click here (Study Completed).
Clinical Data
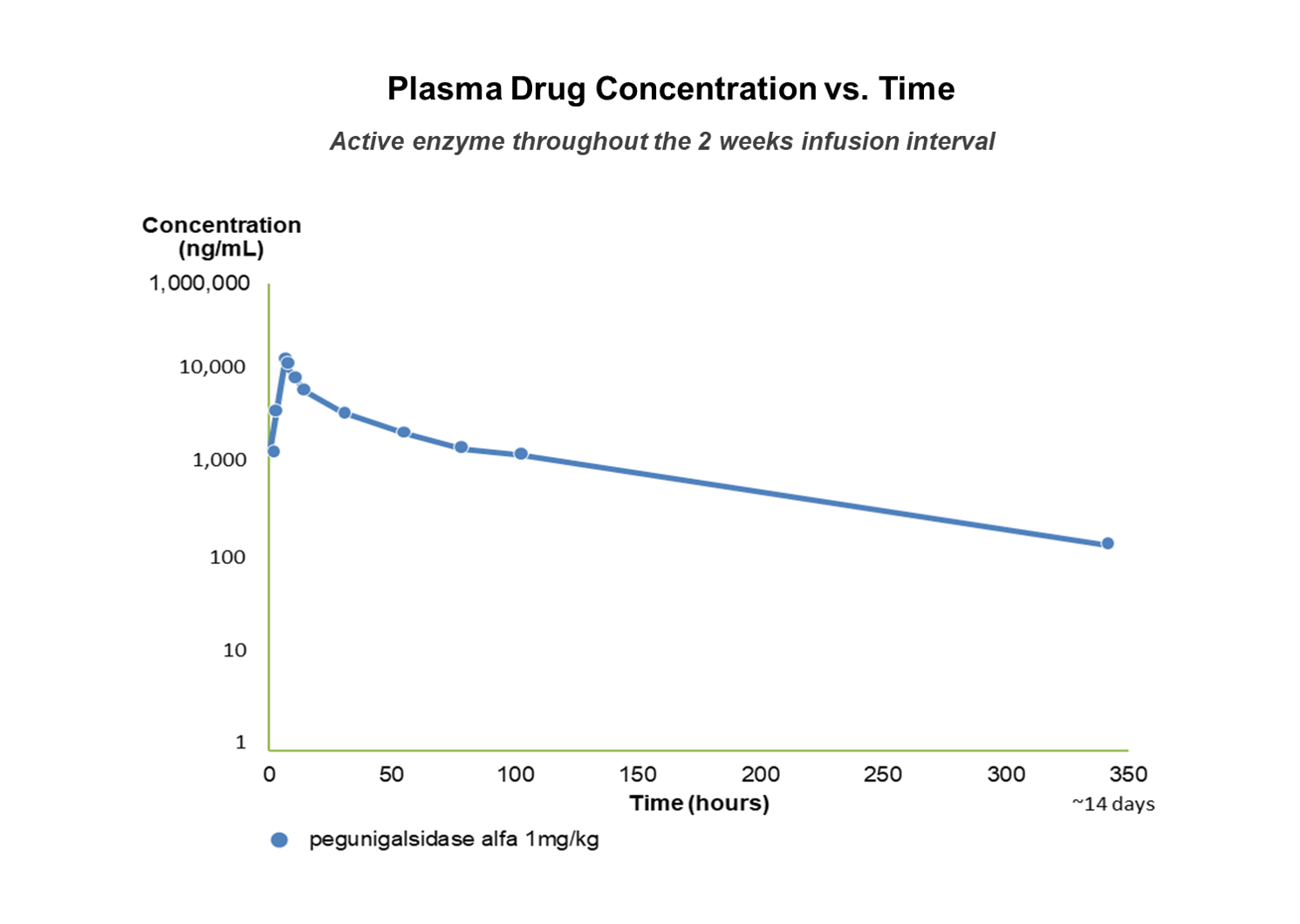

PENGUNIGALSIDASE ALFA – DESIGNED TO PROVIDE 14 DAYS OF ACTIVE ENZYME
In the Phase I/II study, pegunigalsidase alfa has exhibited activity in the plasma for the entire two-week period between infusions1
1 et al, Am. J. Hum. Genet. 68:711–722, 2001
2Schifmann, et al, J Inherit Metab Dis. 2019; 42:534–544.
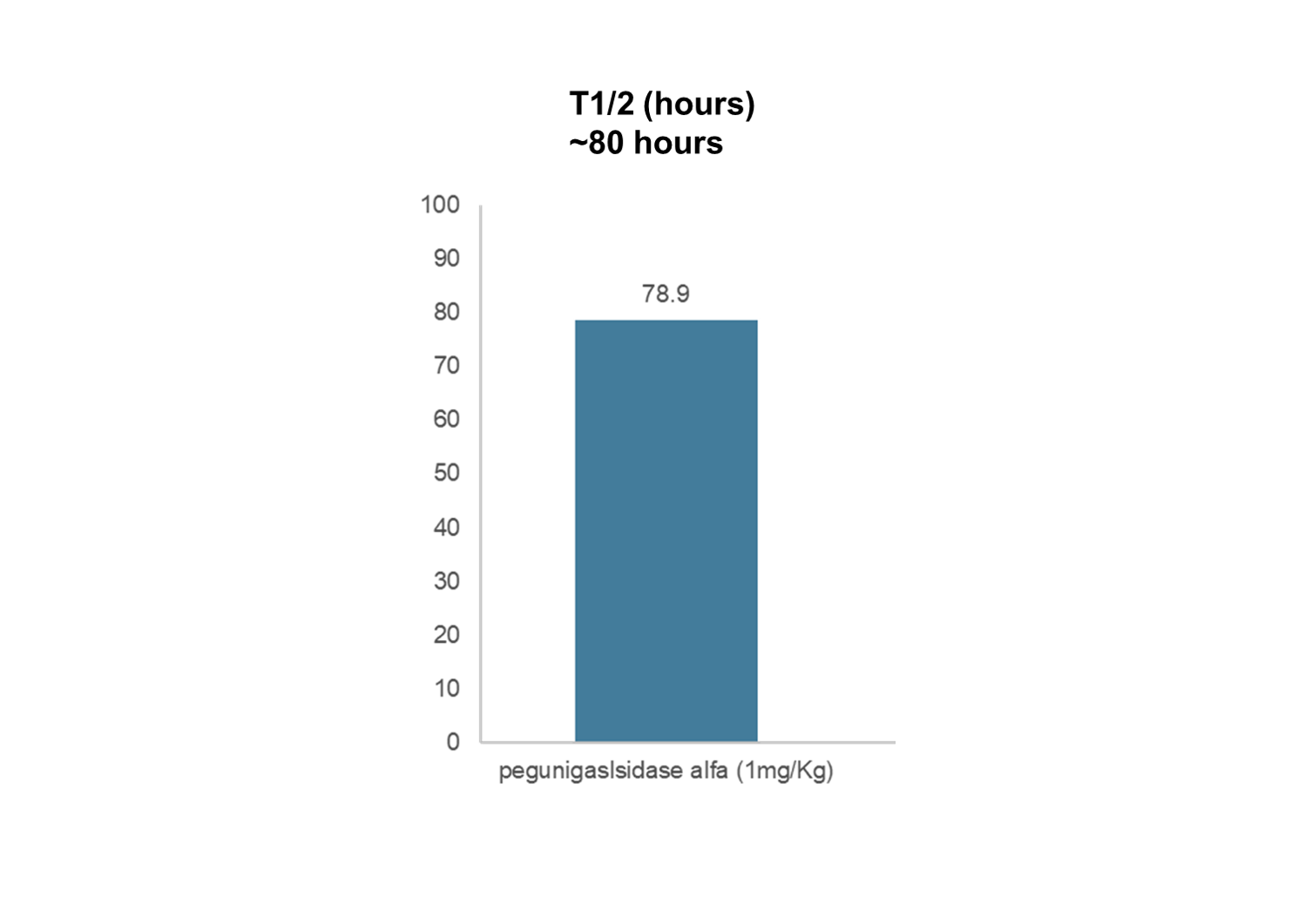

Circulatory Half-Life
In our Phase I/II study, pegunigalsidase alfa has exhibited a half-life of approximately 80 hours. Pegunigalsidase alfa has been observed to be a more stable molecule with a significantly longer circulatory half-life than the currently marketed enzyme replacement therapies for the treatment of Fabry disease, which is approximately 2 hours1
1Schifmann, et al, J Inherit Metab Dis. 2019; 42:534–544
The effect of circulatory half-life has not been established in clinical trials.
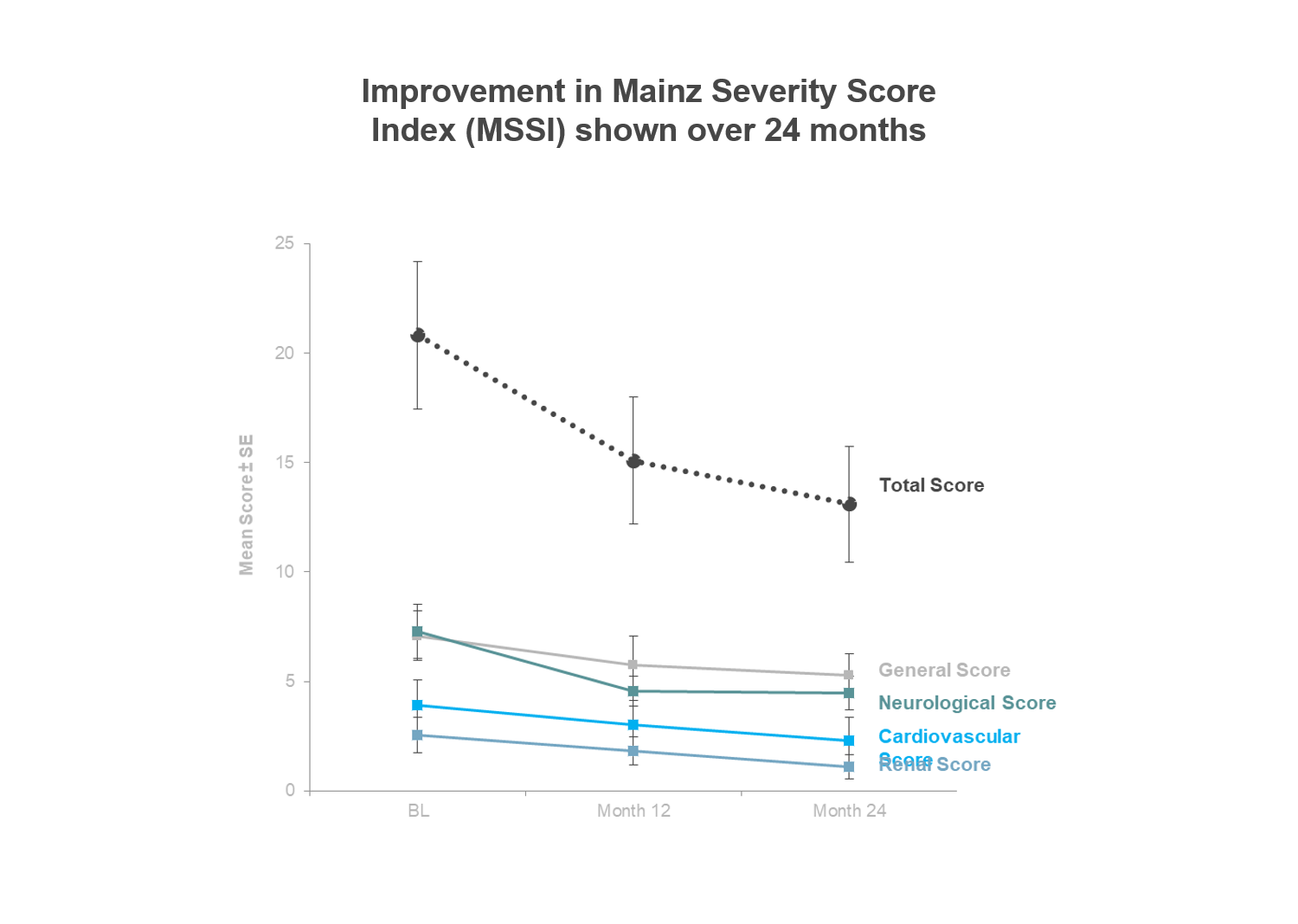

PEGUNIGALSIDASE ALFA – 12 MONTHS AND 24 MONTHS FOLLOW-UP
Two-year follow-up results from our Phase I/II long-term open-label extension trial of pegunigalsidase alfa are available from the 11 patients that were enrolled and treated in the extension trial. The results demonstrate that over a 24-month period, treatment with pegunigalsidase alfa resulted in a continuous reduction in biomarkers for enzymatic activity, continuous stability of renal function, stable cardiac parameters, improvement in gastrointestinal symptoms and a 40% reduction in the Mainz Severity Score Index (MSSI), a means of monitoring disease progression and response to therapy.
Throughout the 24-month follow-up of our Phase I/II long-term open label extension trial, 906 infusions of pegunigalsidase alfa were administered to 16 patients]. This amounts to approximately 35 patient years.
Immunogenicity:
Preliminary immunogenicity results indicate that the unique enzyme characteristics and enhanced PK properties of pegunigalsidase alfa potentially contribute to a low and transient immunogenicity response. Lower immunogenicity means that the drug provokes a lower immune response. In Phase I/II trial, only three of 16 patients (less than 19%) formed anti-drug antibodies (ADA), of which two of these patients (less than 13%) had neutralizing antibodies.
All ADA positive patients were negative for ADA in the second year of treatment.
In our BALANCE study, we plan to assess the level of enzyme activity inhibition among patients that were antibody positive to Fabrazyme®, and the enzyme activity levels in these patients after they are treated with pegunigalsidase alfa.
* Fabrazyme® USPI. Patient characteristics may be different between the pegunigalsidase alfa trials and the Fabrazyme® clinical studies. The development of anti-drug antibodies has not been demonstrated in a head-to-head trial. We plan to evaluate the development of anti-drug antibodies in our Phase 3, head-to-head BALANCE study. For More information on the BALANCE study, please click here
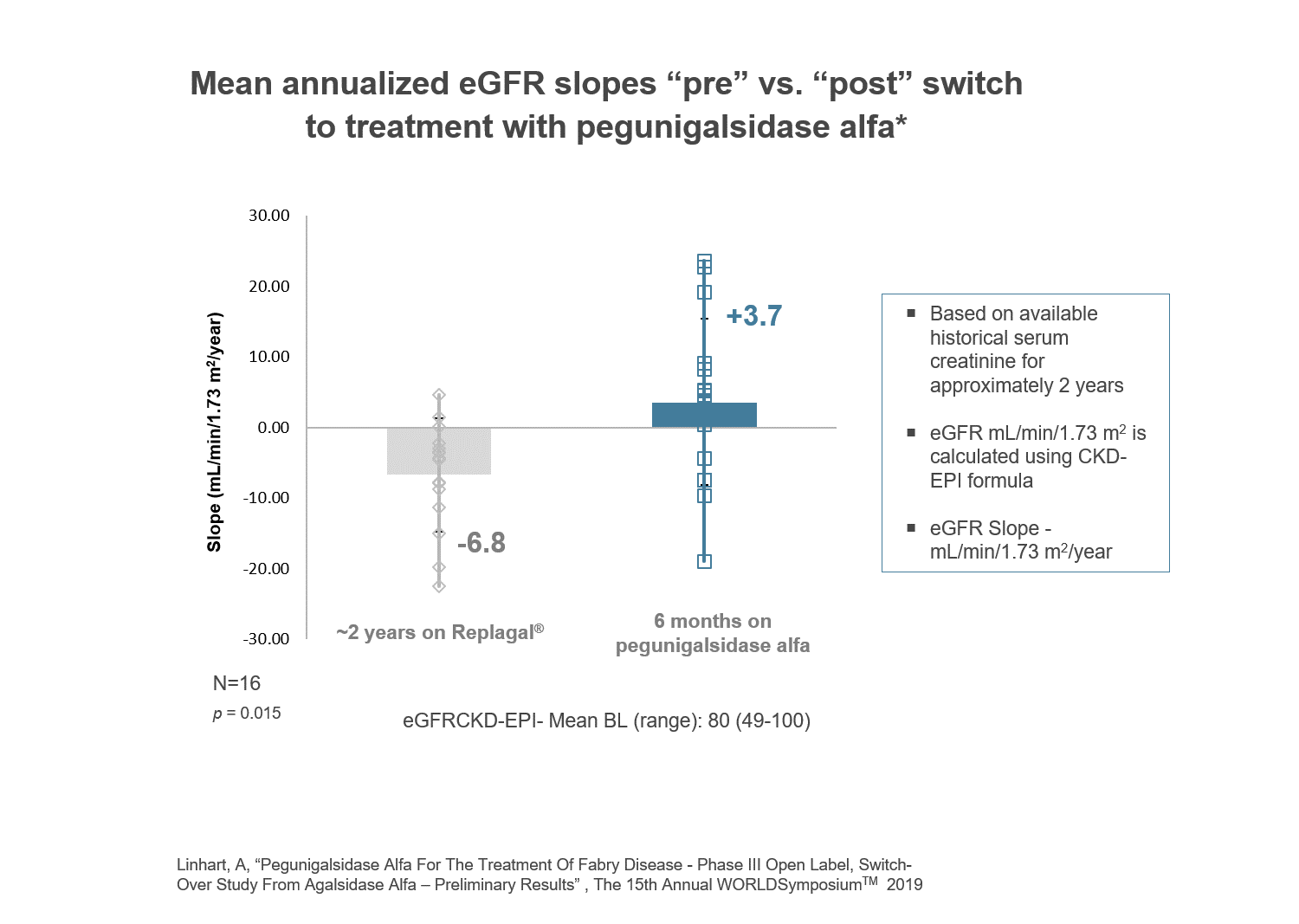

KIDNEY FUNCTION FOLLOWING SWITCH- Interim results
Interim results from 16 patients enrolled in our Phase III BRIDGE study suggest the potential for improved kidney function following switch to pegunigalsidase alfa. . This study remains ongoing and we expect to publish final results when they are available.
See :
BALANCE, BRIDGE and BRIGHT are currently ongoing studies. Results presented are preliminary and interim. Final results are expected to be published when available.
BRIDGE is a single arm switch over study. Improved kidney function has been defined as a secondary endpoint to the study. For more details on the study click here
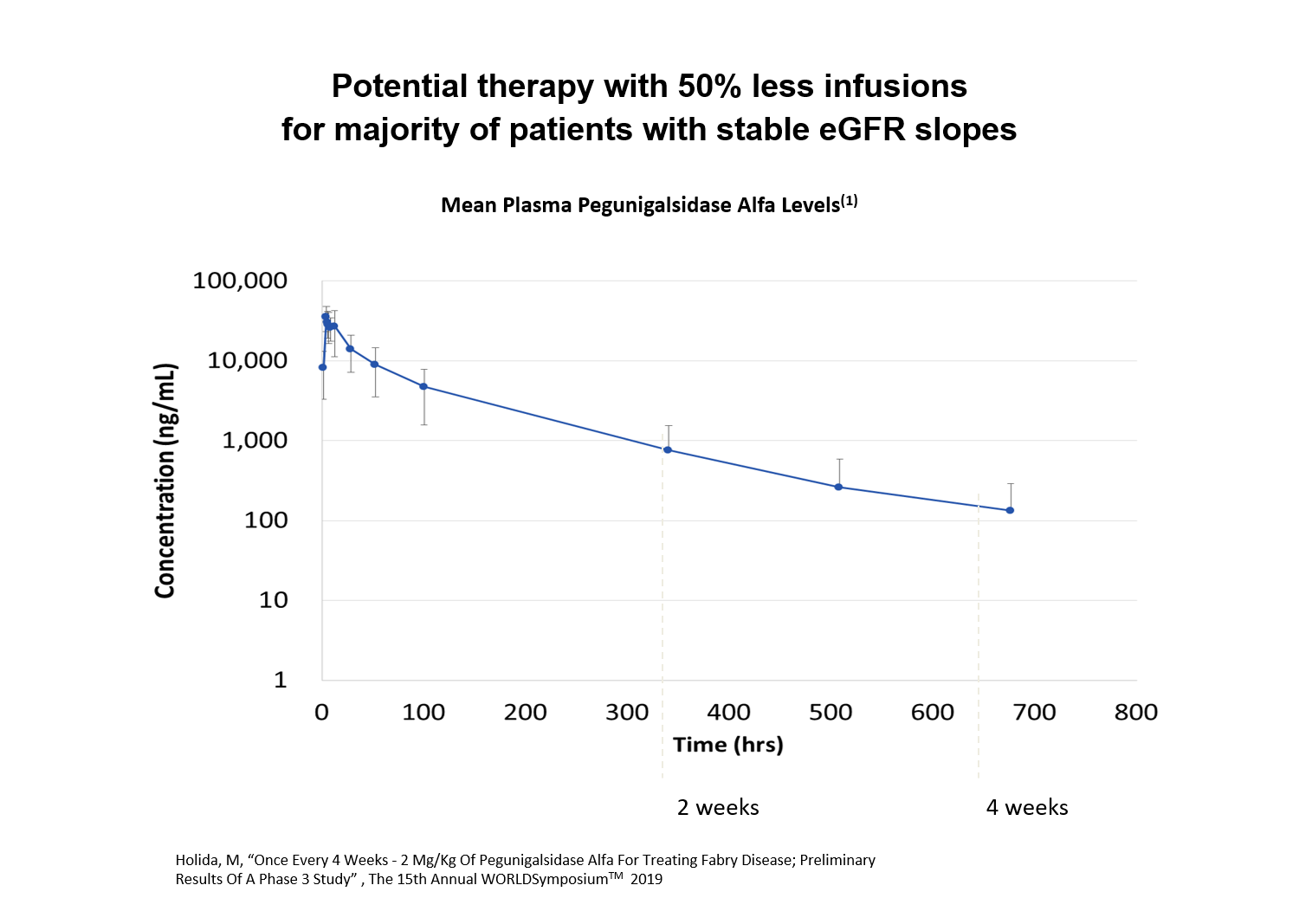

POTENTIAL FOR ONCE-MONTHLY ADMINISTRATION
Pharmacokinetic modeling demonstrates activity of a single infusion of 2mg/kg of pegunigalsidase alfa over a 4-week timeframe.
Based on this modeling, we are evaluating pegunigalsidase alfa for its potential to address the unmet need of less frequent infusions with enzyme replacement therapies and we are conducting the BRIGHT study to explore the safety and efficacy of 2mg/kg of pegunigalsidase alfa every 4 weeks in patients previously treated with Fabrazyme® or Replagal®. Preliminary of the BRIGHT study have demonstrated enzyme activity over 4 weeks.
BALANCE, BRIDGE and BRIGHT are currently ongoing studies. Results presented are preliminary and interim. Final results are expected to be published when available.
BRIGHT is a single arm switch over study. For more details on the study click here
Pivotal Phase III Studies
CLICK HERE TO LEARN MORE ABOUT OUR PHASE III CLINICAL STUDIES
 STUDY COMPLETED
STUDY COMPLETED
The BALANCE study is a randomized, double blind, active controlled study of pegunigalsidase alfa, 1mg/kg infused every two weeks, compared to Fabrazyme® in Fabry patients previously treated with Fabrazyme®. The study has enrolled 78 patients with the objective of the study is to evaluate the effect of pegunigalsidase alfa on renal function by comparison of the eGFR slope (mean annualized change) between treatment groups. Interim results follow up are expected in the first half of 2021 as a basis for European regulatory submission.
Click here for more information about the Balance study
 STUDY COMPLETED
STUDY COMPLETED
The BRIDGE study is an open-label, single-arm switch-over study of 22 patients evaluated at 12 months to assess the efficacy and safety of pegunigalsidase alfa, 1 mg/kg infused every two weeks, in Fabry patients currently treated with Replagal®. This study has been completed.
To learn more about the study results.
 STUDY COMPLETED
STUDY COMPLETED
The BRIGHT study is an open-label switchover study of 30 patients evaluated at 12 months to assess the safety, efficacy and pharmacokinetics of pegunigalsidase alfa 2 mg/kg administered every 4 weeks in Fabry patients previously treated with an approved enzyme replacement therapy: Fabrazyme® or Replagal®. This study has been completed. To learn more on the topline study results.
