ProCellEx® Platform
The first company to gain FDA approval of a protein produced through plant cell-based expression
Protalix’s first commercially available plant cell culture produced protein product, taliglucerase alpha (Elelyso®), was approved by the FDA in May 2012 for long-term enzyme replacement therapy (ERT) for patients with a confirmed diagnosis of type 1 Gaucher disease.
Our Focus
Protalix is dedicated to researching, developing, and marketing recombinant therapeutic proteins with clinically – improved profiles produced by our ProCellEx® plant cell-based protein expression platform.
Our multidisciplinary scientific teams are focused on modifying biological properties of biotherapeutics aimed at improving clinical outcomes of therapies in areas of unmet need. These capabilities are well demonstrated in Protalix’s current pipeline.
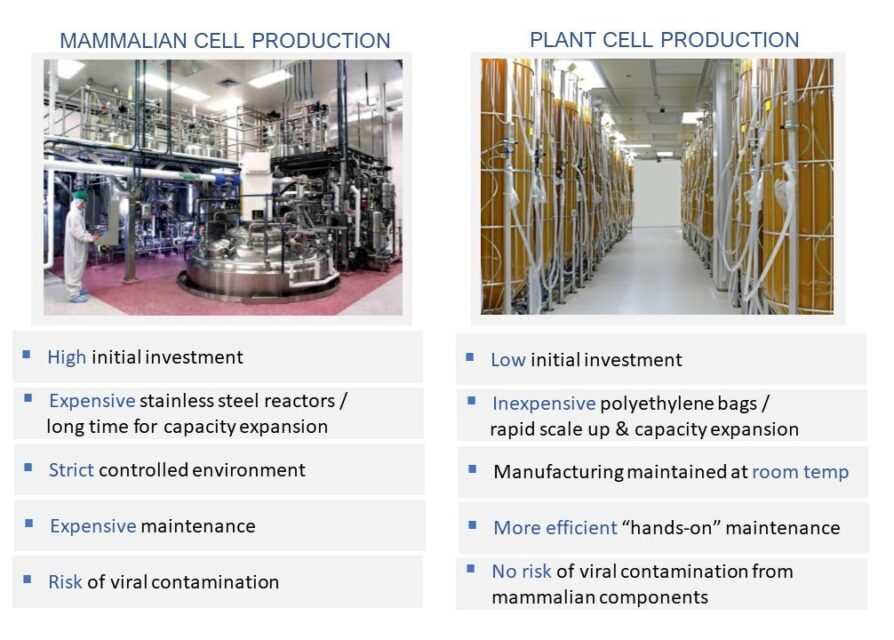
A Proven Alternative to Mammalian Cell-Based Production Technology
Our platform is a proven alternative to the mammalian cell-based production technology (the most common form of recombinant protein production), overcoming many of its weaknesses while offering significant production, regulatory, and cost benefits.
- As opposed to mammalian cells, plant cells tolerate a relatively wide range of culturing conditions and naturally do not carry the risk of infection by human or animal pathogens. This translates into favorable capital investment in the set up as well as in ongoing maintenance of the production plant.
- Plant cells provide a natural barrier to human and animal pathogens and are able to utilize a simple culture medium with no requirement to any animal – originated supplements. This may prove to be important advantage from a regulatory perspective.
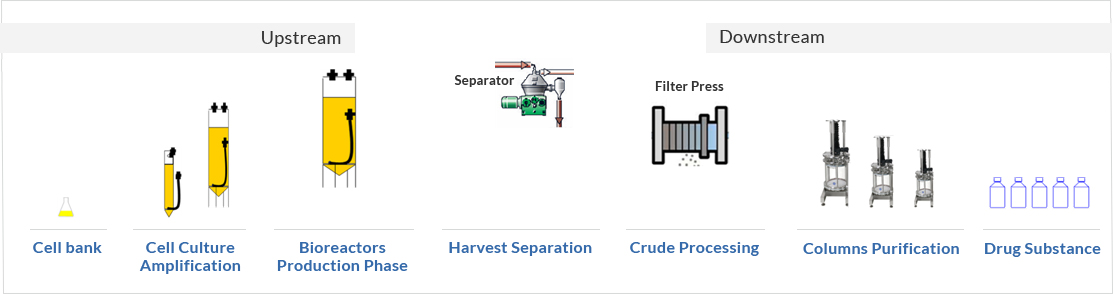
- Protalix’s ProCellEx® platform uses flexible polyethylene disposable bioreactors designed and optimized for plant cell cultures. As opposed to the large stainless steel reactors commonly used for recombinant protein production, the ProCellEx® bioreactors are easy to use, entail advantageous initial capital investment, and can be scalable cost-effectively. Additionally, maintenance of the bioreators between cycles may be performed more efficiently.
- ProCellEx® can potentially express certain proteins that are difficult to express in other systems.
- Through the ProCellEx® system, it is possible in certain cases to develop and commercialize recombinant proteins without infringing upon the method-based patents or other intellectual property rights of third parties.
- Additionally, the ProCellEx® platform has the ability to more rapidly develop clinical material for testing versus other protein platforms.
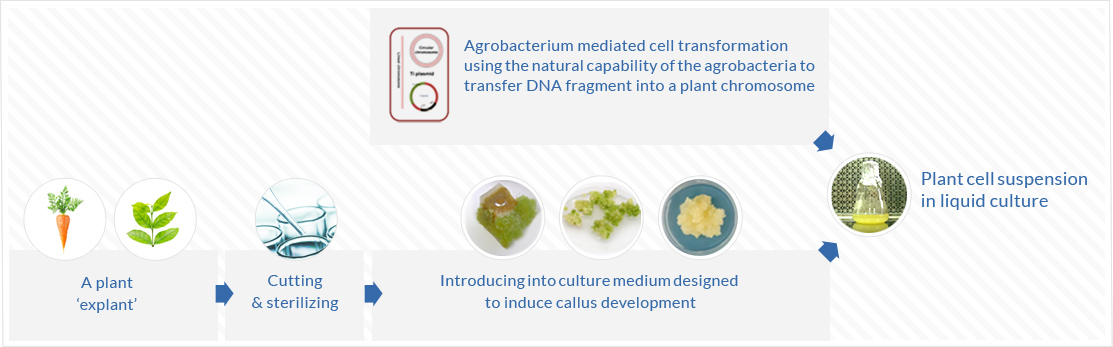
Development of transgenic cell lines for production of target protein
Process overview